The industry association Spectaris says that "a new era has dawned for manufacturers of medical devices in the EU".
Many new requirements must be met by manufacturers, such as:
- Application of an electronic European vigilance and market surveillance system with shorter reporting deadlines
- Significantly higher documentation requirements: e.g. Post-market surveillance plan, periodic safety update report (Class IIa and above), post-market clinical surveillance plan and assessment report
- Introduction of a European traceability system: Unique Device Identification (UDI)
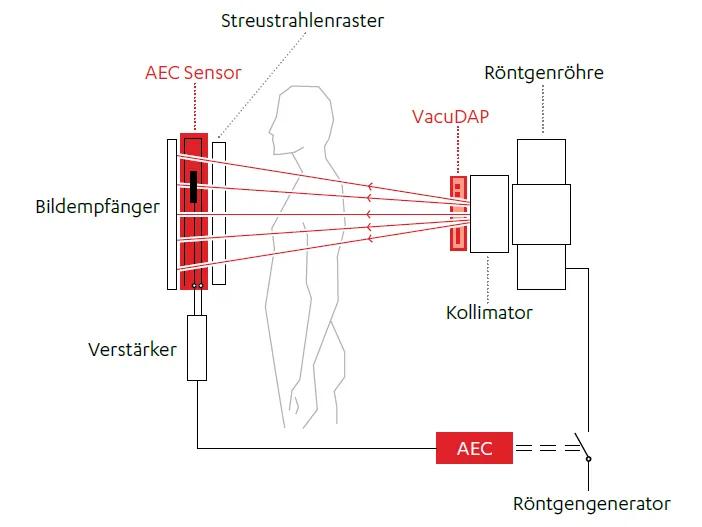
Modern medicine faces the challenge of making diagnostic imaging procedures as safe as possible. A key aspect of this is minimizing patient exposure to radiation. VacuTec contributes to this with its VacuDAP dose area product measuring devices and the automatic exposure chambers (BAK or AEC sensor). As accessories to the X-ray device, VacuDAP and BAK are medical devices and must therefore be newly approved in accordance with the MDR; the previous approval in accordance with the old legislation under MDD 93/42/EEC (Medical Device Directive) had a transitional period until 2024. Due to the complex re-approval process and the overload of the approval bodies, this transitional period was extended by the EU Commission until 2028 in order to prevent acute bottlenecks in the supply of medical devices in the EU.
Parallel to the introduction of UDI labeling and retesting in accordance with the latest safety and EMC standards, VacuTec commissioned MDR approval from TÜV Rheinland at the beginning of 2022. In summer 2023, the technical documentation comprising around 250 different documents was submitted to the BAK for review, which was then successfully completed with several rounds of testing by summer 2024. Another challenge was the one-week MDR audit in November 2024, which was passed without any deviations. The official certificate is to be issued by QII/2025.
"We are proud of the commitment and hard work of our team that made this success possible," says Managing Director Dr. Bettina Jakob. "Compliance with the MDR requirements is not only a regulatory obligation for us, but also a further step in ensuring the safety and satisfaction of our customers."
The MDR approval marks an important milestone for VacuTec and underlines the company's continuous efforts to meet the highest quality standards and offer innovative solutions in the field of medical technology. VacuTec sets standards in radiation measurement technology and contributes significantly to improving patient safety in diagnostic imaging. With advanced technologies and a clear focus on radiation protection, the company offers solutions that both meet the high demands of modern medicine and ensure the protection of patients.